Sbírka 79+ Structure Of Atom Of Sodium Vynikající
Sbírka 79+ Structure Of Atom Of Sodium Vynikající. Sodium, like every reactive element, is never found free in nature. It has a total of \11\ protons and \11\ electrons. Sodium is a soft, bright, silvery metal which floats on water. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The valence shell of the sodium atom is \3\.
Prezentováno Sodium Atomic Structure Stock Image C023 2461 Science Photo Library
It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. The electronic configuration of sodium is: Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The valence shell of the sodium atom is \3\. Sodium is a soft, bright, silvery metal which floats on water.Sodium is a soft, bright, silvery metal which floats on water.
It has a total of \11\ protons and \11\ electrons. It normally does not ignite in air at temperatures below 115°c. It has a total of \11\ protons and \11\ electrons. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium, like every reactive element, is never found free in nature.

Sodium is a soft, bright, silvery metal which floats on water. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,. The rings represents electron shells and the 'x' represents the electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. The 'na' which is the word at the center of the ring is the atom/element. Sodium is a soft, bright, silvery metal which floats on water.
It normally does not ignite in air at temperatures below 115°c. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The rings represents electron shells and the 'x' represents the electrons. Sodium, like every reactive element, is never found free in nature. The valence shell of the sodium atom is \3\. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It has a total of \11\ protons and \11\ electrons. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium, like every reactive element, is never found free in nature. Sodium is a soft, bright, silvery metal which floats on water. The rings represents electron shells and the 'x' represents the electrons. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.
15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. . It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.

Sodium, like every reactive element, is never found free in nature. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. It has a total of \11\ protons and \11\ electrons. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The 'na' which is the word at the center of the ring is the atom/element. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The electronic configuration of sodium is:. Sodium is a soft, bright, silvery metal which floats on water.

Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The valence shell of the sodium atom is \3\. The rings represents electron shells and the 'x' represents the electrons. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. In this video we'll look at the atomic structure, valence electrons,. Sodium, like every reactive element, is never found free in nature. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is a soft, bright, silvery metal which floats on water. The 'na' which is the word at the center of the ring is the atom/element.
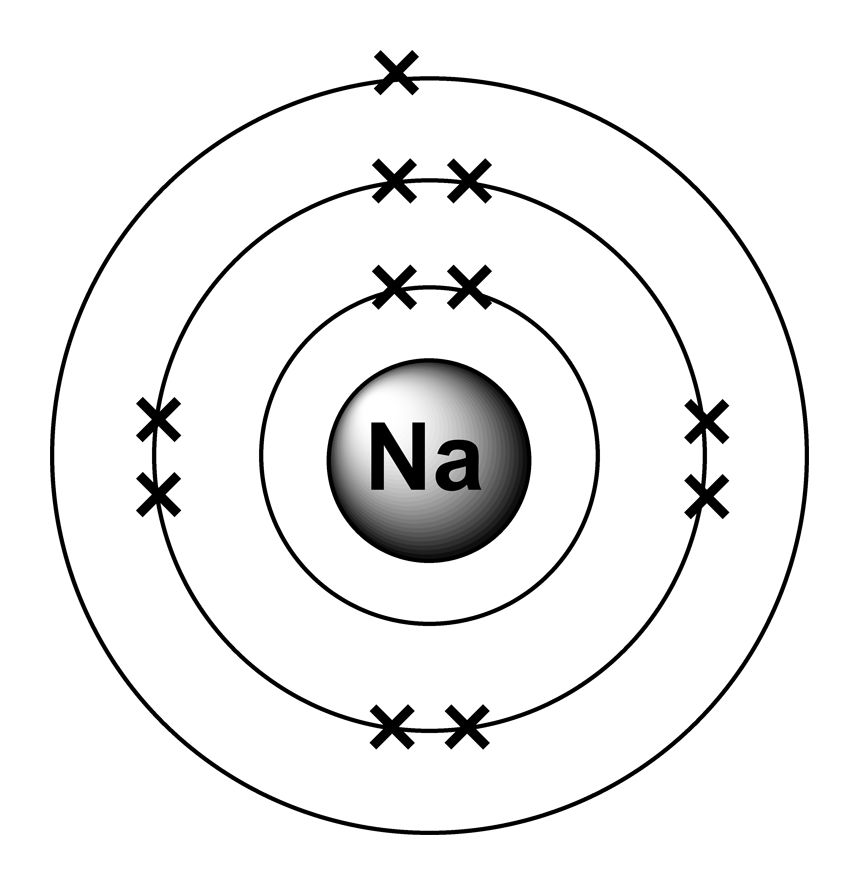
Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The rings represents electron shells and the 'x' represents the electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water... The valence shell of the sodium atom is \3\.

Sodium is a soft, bright, silvery metal which floats on water. The valence shell of the sodium atom is \3\. The 'na' which is the word at the center of the ring is the atom/element. The electronic configuration of sodium is: In this video we'll look at the atomic structure, valence electrons,. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It normally does not ignite in air at temperatures below 115°c. It has a total of \11\ protons and \11\ electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium is a soft, bright, silvery metal which floats on water. The rings represents electron shells and the 'x' represents the electrons... Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.

It has a total of \11\ protons and \11\ electrons.. It has a total of \11\ protons and \11\ electrons. The electronic configuration of sodium is: It normally does not ignite in air at temperatures below 115°c. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.. Sodium, like every reactive element, is never found free in nature.

The 'na' which is the word at the center of the ring is the atom/element... The 'na' which is the word at the center of the ring is the atom/element. The valence shell of the sodium atom is \3\. It has a total of \11\ protons and \11\ electrons. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It normally does not ignite in air at temperatures below 115°c.

It normally does not ignite in air at temperatures below 115°c... It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. It has a total of \11\ protons and \11\ electrons. In this video we'll look at the atomic structure, valence electrons,. Sodium is a soft, bright, silvery metal which floats on water. The electronic configuration of sodium is: Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element.. The rings represents electron shells and the 'x' represents the electrons.

Sodium, like every reactive element, is never found free in nature.. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It has a total of \11\ protons and \11\ electrons. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium, like every reactive element, is never found free in nature.. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It has a total of \11\ protons and \11\ electrons. Sodium is a soft, bright, silvery metal which floats on water. The valence shell of the sodium atom is \3\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.. It has a total of \11\ protons and \11\ electrons.

It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.. It has a total of \11\ protons and \11\ electrons. The electronic configuration of sodium is: Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,. The valence shell of the sodium atom is \3\. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring.

Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The valence shell of the sodium atom is \3\.. The 'na' which is the word at the center of the ring is the atom/element.

It has a total of \11\ protons and \11\ electrons. The rings represents electron shells and the 'x' represents the electrons.

In this video we'll look at the atomic structure, valence electrons,... It normally does not ignite in air at temperatures below 115°c. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.. The 'na' which is the word at the center of the ring is the atom/element.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It normally does not ignite in air at temperatures below 115°c. Sodium, like every reactive element, is never found free in nature... The valence shell of the sodium atom is \3\.

Sodium is a soft, bright, silvery metal which floats on water.. In this video we'll look at the atomic structure, valence electrons,. The rings represents electron shells and the 'x' represents the electrons. It normally does not ignite in air at temperatures below 115°c. It has a total of \11\ protons and \11\ electrons.. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.

Sodium is a soft, bright, silvery metal which floats on water. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. It normally does not ignite in air at temperatures below 115°c. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It has a total of \11\ protons and \11\ electrons. The valence shell of the sodium atom is \3\. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element. Sodium, like every reactive element, is never found free in nature. The electronic configuration of sodium is: The rings represents electron shells and the 'x' represents the electrons. The electronic configuration of sodium is:

It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The electronic configuration of sodium is: The 'na' which is the word at the center of the ring is the atom/element. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It normally does not ignite in air at temperatures below 115°c... The rings represents electron shells and the 'x' represents the electrons.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The 'na' which is the word at the center of the ring is the atom/element. The rings represents electron shells and the 'x' represents the electrons. Sodium, like every reactive element, is never found free in nature. Sodium is a soft, bright, silvery metal which floats on water... Sodium is a soft, bright, silvery metal which floats on water.

The electronic configuration of sodium is: It has a total of \11\ protons and \11\ electrons. In this video we'll look at the atomic structure, valence electrons,. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The electronic configuration of sodium is: It normally does not ignite in air at temperatures below 115°c.

In this video we'll look at the atomic structure, valence electrons,... Sodium, like every reactive element, is never found free in nature. The valence shell of the sodium atom is \3\.. The valence shell of the sodium atom is \3\.

Sodium, like every reactive element, is never found free in nature.. . 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry.
It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It normally does not ignite in air at temperatures below 115°c. In this video we'll look at the atomic structure, valence electrons,. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It has a total of \11\ protons and \11\ electrons. The rings represents electron shells and the 'x' represents the electrons. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring.

The valence shell of the sodium atom is \3\. Sodium, like every reactive element, is never found free in nature. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. In this video we'll look at the atomic structure, valence electrons,. It normally does not ignite in air at temperatures below 115°c. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium is a soft, bright, silvery metal which floats on water.

The electronic configuration of sodium is: The rings represents electron shells and the 'x' represents the electrons. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The 'na' which is the word at the center of the ring is the atom/element. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry.. Sodium is a soft, bright, silvery metal which floats on water.

The 'na' which is the word at the center of the ring is the atom/element.. Sodium, like every reactive element, is never found free in nature... In this video we'll look at the atomic structure, valence electrons,.

The 'na' which is the word at the center of the ring is the atom/element. The valence shell of the sodium atom is \3\.

The rings represents electron shells and the 'x' represents the electrons. Sodium is a soft, bright, silvery metal which floats on water. It normally does not ignite in air at temperatures below 115°c. Sodium, like every reactive element, is never found free in nature. The electronic configuration of sodium is: Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. In this video we'll look at the atomic structure, valence electrons,. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry... The valence shell of the sodium atom is \3\.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.. Sodium is a soft, bright, silvery metal which floats on water. The rings represents electron shells and the 'x' represents the electrons. In this video we'll look at the atomic structure, valence electrons,. The valence shell of the sodium atom is \3\. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.. The electronic configuration of sodium is:

The electronic configuration of sodium is: The valence shell of the sodium atom is \3\... Sodium is a soft, bright, silvery metal which floats on water.

Sodium is a soft, bright, silvery metal which floats on water... It has a total of \11\ protons and \11\ electrons. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium is a soft, bright, silvery metal which floats on water. The valence shell of the sodium atom is \3\. The rings represents electron shells and the 'x' represents the electrons. The 'na' which is the word at the center of the ring is the atom/element. The electronic configuration of sodium is: Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.. Sodium, like every reactive element, is never found free in nature.

It has a total of \11\ protons and \11\ electrons. The rings represents electron shells and the 'x' represents the electrons. It normally does not ignite in air at temperatures below 115°c. Sodium is a soft, bright, silvery metal which floats on water. Sodium, like every reactive element, is never found free in nature. The valence shell of the sodium atom is \3\.. In this video we'll look at the atomic structure, valence electrons,.

15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The valence shell of the sodium atom is \3\. Sodium is a soft, bright, silvery metal which floats on water. In this video we'll look at the atomic structure, valence electrons,. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The rings represents electron shells and the 'x' represents the electrons. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry... The rings represents electron shells and the 'x' represents the electrons.

Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The electronic configuration of sodium is: 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. The rings represents electron shells and the 'x' represents the electrons. Sodium is a soft, bright, silvery metal which floats on water. In this video we'll look at the atomic structure, valence electrons,. It has a total of \11\ protons and \11\ electrons.. Sodium is a soft, bright, silvery metal which floats on water.

It normally does not ignite in air at temperatures below 115°c. The rings represents electron shells and the 'x' represents the electrons. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium is a soft, bright, silvery metal which floats on water.. It normally does not ignite in air at temperatures below 115°c.

Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element. In this video we'll look at the atomic structure, valence electrons,. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It normally does not ignite in air at temperatures below 115°c. Sodium is a soft, bright, silvery metal which floats on water.. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\.

Sodium, like every reactive element, is never found free in nature. The rings represents electron shells and the 'x' represents the electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry.
The 'na' which is the word at the center of the ring is the atom/element. In this video we'll look at the atomic structure, valence electrons,. Sodium, like every reactive element, is never found free in nature. The 'na' which is the word at the center of the ring is the atom/element. It has a total of \11\ protons and \11\ electrons. It normally does not ignite in air at temperatures below 115°c. The electronic configuration of sodium is: It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry.
Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide... Sodium is a soft, bright, silvery metal which floats on water. It normally does not ignite in air at temperatures below 115°c. The valence shell of the sodium atom is \3\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The rings represents electron shells and the 'x' represents the electrons. The electronic configuration of sodium is:.. It has a total of \11\ protons and \11\ electrons.

Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. It has a total of \11\ protons and \11\ electrons. The rings represents electron shells and the 'x' represents the electrons. The 'na' which is the word at the center of the ring is the atom/element. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.

The 'na' which is the word at the center of the ring is the atom/element.. It normally does not ignite in air at temperatures below 115°c. Sodium, like every reactive element, is never found free in nature. The valence shell of the sodium atom is \3\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. The rings represents electron shells and the 'x' represents the electrons.

Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The valence shell of the sodium atom is \3\. Sodium, like every reactive element, is never found free in nature. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.. It normally does not ignite in air at temperatures below 115°c.

Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The valence shell of the sodium atom is \3\. The rings represents electron shells and the 'x' represents the electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium is a soft, bright, silvery metal which floats on water. It normally does not ignite in air at temperatures below 115°c.. It normally does not ignite in air at temperatures below 115°c.

Sodium, like every reactive element, is never found free in nature... Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. The valence shell of the sodium atom is \3\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. The rings represents electron shells and the 'x' represents the electrons.. The valence shell of the sodium atom is \3\.

In this video we'll look at the atomic structure, valence electrons,.. In this video we'll look at the atomic structure, valence electrons,. The valence shell of the sodium atom is \3\. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. The electronic configuration of sodium is: The rings represents electron shells and the 'x' represents the electrons.. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.

It has a total of \11\ protons and \11\ electrons.. The valence shell of the sodium atom is \3\. Sodium is a soft, bright, silvery metal which floats on water. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium, like every reactive element, is never found free in nature. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. It has a total of \11\ protons and \11\ electrons. In this video we'll look at the atomic structure, valence electrons,... The valence shell of the sodium atom is \3\.

The rings represents electron shells and the 'x' represents the electrons.. It normally does not ignite in air at temperatures below 115°c. The electronic configuration of sodium is: The valence shell of the sodium atom is \3\. The 'na' which is the word at the center of the ring is the atom/element. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. In this video we'll look at the atomic structure, valence electrons,. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring.. The valence shell of the sodium atom is \3\.

It normally does not ignite in air at temperatures below 115°c. The rings represents electron shells and the 'x' represents the electrons. Sodium is a soft, bright, silvery metal which floats on water. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. The electronic configuration of sodium is: Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,. The valence shell of the sodium atom is \3\... Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.

The 'na' which is the word at the center of the ring is the atom/element.. It normally does not ignite in air at temperatures below 115°c. Sodium, like every reactive element, is never found free in nature. The electronic configuration of sodium is: Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.
Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide.. It normally does not ignite in air at temperatures below 115°c.. The valence shell of the sodium atom is \3\.

Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The 'na' which is the word at the center of the ring is the atom/element. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The rings represents electron shells and the 'x' represents the electrons... It has a total of \11\ protons and \11\ electrons.

Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. In this video we'll look at the atomic structure, valence electrons,. The 'na' which is the word at the center of the ring is the atom/element. Sodium is a soft, bright, silvery metal which floats on water. Sodium, like every reactive element, is never found free in nature. The rings represents electron shells and the 'x' represents the electrons. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. It normally does not ignite in air at temperatures below 115°c. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The electronic configuration of sodium is:. The valence shell of the sodium atom is \3\.

The rings represents electron shells and the 'x' represents the electrons. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is a soft, bright, silvery metal which floats on water. The rings represents electron shells and the 'x' represents the electrons. In this video we'll look at the atomic structure, valence electrons,. Sodium, like every reactive element, is never found free in nature. It has a total of \11\ protons and \11\ electrons. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element. It normally does not ignite in air at temperatures below 115°c. The electronic configuration of sodium is: Sodium, like every reactive element, is never found free in nature.

The 'na' which is the word at the center of the ring is the atom/element. The electronic configuration of sodium is: Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium, like every reactive element, is never found free in nature. The 'na' which is the word at the center of the ring is the atom/element. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring.. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.

The 'na' which is the word at the center of the ring is the atom/element. Sodium, like every reactive element, is never found free in nature. It normally does not ignite in air at temperatures below 115°c. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The rings represents electron shells and the 'x' represents the electrons. It has a total of \11\ protons and \11\ electrons. In this video we'll look at the atomic structure, valence electrons,. Sodium is a soft, bright, silvery metal which floats on water. The valence shell of the sodium atom is \3\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry.. In this video we'll look at the atomic structure, valence electrons,.

Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. . It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water.

Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. In this video we'll look at the atomic structure, valence electrons,. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring. It has a total of \11\ protons and \11\ electrons. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. The rings represents electron shells and the 'x' represents the electrons. The electronic configuration of sodium is: The valence shell of the sodium atom is \3\. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,.
Sodium, like every reactive element, is never found free in nature. Sodium atom have 11 electrons ,thus we have to draw 3 rings around the word'na' and the 'x' on each of the ring.

It normally does not ignite in air at temperatures below 115°c... Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. The 'na' which is the word at the center of the ring is the atom/element. It has a total of \11\ protons and \11\ electrons. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. The electronic configuration of sodium is: Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. Sodium is a soft, bright, silvery metal which floats on water.

Sodium, like every reactive element, is never found free in nature. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. Sodium is a soft, bright, silvery metal which floats on water. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. Sodium, like every reactive element, is never found free in nature. It normally does not ignite in air at temperatures below 115°c. In this video we'll look at the atomic structure, valence electrons,.. In this video we'll look at the atomic structure, valence electrons,.

It normally does not ignite in air at temperatures below 115°c. Sodium, like every reactive element, is never found free in nature. In this video we'll look at the atomic structure, valence electrons,. The electronic configuration of sodium is: It normally does not ignite in air at temperatures below 115°c. The 'na' which is the word at the center of the ring is the atom/element.. The electronic configuration of sodium is:

Sodium, like every reactive element, is never found free in nature... It normally does not ignite in air at temperatures below 115°c. Decomposition in water results in the evolution of hydrogen and the formation of the hydroxide. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. Sodium is an atom in the periodic table with atomic number \11\ and mass number\23\. 15.10.2020 · the sodium atom (na) is commonly used for examples and practice problems in chemistry. The valence shell of the sodium atom is \3\.
