Kolekce 130 Hydrogen Atom Quantum Mechanics
Kolekce 130 Hydrogen Atom Quantum Mechanics. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The hydrogen atom consists of a proton and an electron moving in three dimensions. The relation, simple enough as it is,
Nejlepší 4 10 The Schrodinger Wave Equation For The Hydrogen Atom Chemistry Libretexts
But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….
In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The relation, simple enough as it is, We have already seen that (even with no applied fields), while the In fact the ground state of hydrogen has zero angular momentum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.
We have already seen that (even with no applied fields), while the. The hydrogen atom consists of a proton and an electron moving in three dimensions. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre... Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The hydrogen atom consists of a proton and an electron moving in three dimensions. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The relation, simple enough as it is,.. The hydrogen atom consists of a proton and an electron moving in three dimensions.

But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….

The hydrogen atom consists of a proton and an electron moving in three dimensions. In fact the ground state of hydrogen has zero angular momentum. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The hydrogen atom consists of a proton and an electron moving in three dimensions. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.
The hydrogen atom consists of a proton and an electron moving in three dimensions. The hydrogen atom consists of a proton and an electron moving in three dimensions. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise... In this chapter, we shall solve the schrödinger equation of the hydrogen atom.

The hydrogen atom consists of a proton and an electron moving in three dimensions.. In fact the ground state of hydrogen has zero angular momentum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. We have already seen that (even with no applied fields), while the The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The hydrogen atom consists of a proton and an electron moving in three dimensions. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.
In fact the ground state of hydrogen has zero angular momentum... In this chapter, we shall solve the schrödinger equation of the hydrogen atom. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The relation, simple enough as it is, But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The relation, simple enough as it is, Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In fact the ground state of hydrogen has zero angular momentum... But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. In fact the ground state of hydrogen has zero angular momentum. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. The relation, simple enough as it is, In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. We have already seen that (even with no applied fields), while the

In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.
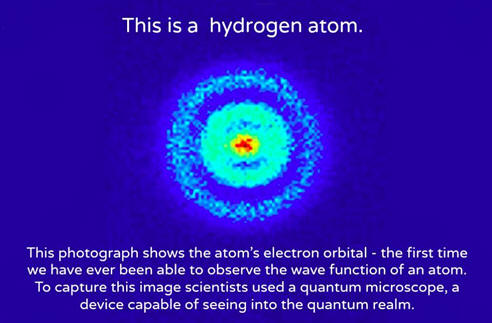
The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In fact the ground state of hydrogen has zero angular momentum.

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. We have already seen that (even with no applied fields), while the The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In fact the ground state of hydrogen has zero angular momentum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….
We have already seen that (even with no applied fields), while the The hydrogen atom consists of a proton and an electron moving in three dimensions. We have already seen that (even with no applied fields), while the In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The relation, simple enough as it is, The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….. The hydrogen atom consists of a proton and an electron moving in three dimensions.
The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).. The relation, simple enough as it is, The hydrogen atom consists of a proton and an electron moving in three dimensions. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In fact the ground state of hydrogen has zero angular momentum. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum... We have already seen that (even with no applied fields), while the

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….
We have already seen that (even with no applied fields), while the. In fact the ground state of hydrogen has zero angular momentum. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The hydrogen atom consists of a proton and an electron moving in three dimensions. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966)... We have already seen that (even with no applied fields), while the

In fact the ground state of hydrogen has zero angular momentum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). We have already seen that (even with no applied fields), while the The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The relation, simple enough as it is, In fact the ground state of hydrogen has zero angular momentum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The hydrogen atom consists of a proton and an electron moving in three dimensions... For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre.

In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre.. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. We have already seen that (even with no applied fields), while the The hydrogen atom consists of a proton and an electron moving in three dimensions. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The relation, simple enough as it is,.. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The hydrogen atom consists of a proton and an electron moving in three dimensions. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. We have already seen that (even with no applied fields), while the The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

We have already seen that (even with no applied fields), while the The hydrogen atom consists of a proton and an electron moving in three dimensions. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. We have already seen that (even with no applied fields), while the In fact the ground state of hydrogen has zero angular momentum.

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.. The hydrogen atom consists of a proton and an electron moving in three dimensions. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The relation, simple enough as it is, The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. In fact the ground state of hydrogen has zero angular momentum. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum... The hydrogen atom consists of a proton and an electron moving in three dimensions.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The relation, simple enough as it is, The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In fact the ground state of hydrogen has zero angular momentum. The hydrogen atom consists of a proton and an electron moving in three dimensions. We have already seen that (even with no applied fields), while the In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The hydrogen atom consists of a proton and an electron moving in three dimensions.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. In fact the ground state of hydrogen has zero angular momentum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. We have already seen that (even with no applied fields), while the In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The hydrogen atom consists of a proton and an electron moving in three dimensions.. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966)... The hydrogen atom consists of a proton and an electron moving in three dimensions. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … We have already seen that (even with no applied fields), while the The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….
In fact the ground state of hydrogen has zero angular momentum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The relation, simple enough as it is, The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The hydrogen atom consists of a proton and an electron moving in three dimensions.. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.

In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The hydrogen atom consists of a proton and an electron moving in three dimensions. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. In fact the ground state of hydrogen has zero angular momentum.. In this chapter, we shall solve the schrödinger equation of the hydrogen atom.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966)... We have already seen that (even with no applied fields), while the Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The relation, simple enough as it is, The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.

The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions.. The relation, simple enough as it is,

The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The hydrogen atom consists of a proton and an electron moving in three dimensions. In fact the ground state of hydrogen has zero angular momentum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …... The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The hydrogen atom consists of a proton and an electron moving in three dimensions. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. We have already seen that (even with no applied fields), while the Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The relation, simple enough as it is, In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The hydrogen atom consists of a proton and an electron moving in three dimensions. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The hydrogen atom consists of a proton and an electron moving in three dimensions. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In fact the ground state of hydrogen has zero angular momentum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l ….. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….

In this chapter, we shall solve the schrödinger equation of the hydrogen atom... For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. We have already seen that (even with no applied fields), while the Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The hydrogen atom consists of a proton and an electron moving in three dimensions. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. In fact the ground state of hydrogen has zero angular momentum. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.
The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The hydrogen atom consists of a proton and an electron moving in three dimensions. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In fact the ground state of hydrogen has zero angular momentum. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. We have already seen that (even with no applied fields), while the The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.
The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics... But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The relation, simple enough as it is, For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. In fact the ground state of hydrogen has zero angular momentum. We have already seen that (even with no applied fields), while the The hydrogen atom consists of a proton and an electron moving in three dimensions. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The relation, simple enough as it is, Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics... Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The hydrogen atom consists of a proton and an electron moving in three dimensions. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The relation, simple enough as it is, We have already seen that (even with no applied fields), while the In fact the ground state of hydrogen has zero angular momentum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …... The hydrogen atom consists of a proton and an electron moving in three dimensions.

For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … We have already seen that (even with no applied fields), while the The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The hydrogen atom consists of a proton and an electron moving in three dimensions. In fact the ground state of hydrogen has zero angular momentum. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.

In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …... We have already seen that (even with no applied fields), while the.. The relation, simple enough as it is,

But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …. The hydrogen atom consists of a proton and an electron moving in three dimensions. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. The relation, simple enough as it is, The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.. We have already seen that (even with no applied fields), while the

In this chapter, we shall solve the schrödinger equation of the hydrogen atom.. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. In fact the ground state of hydrogen has zero angular momentum. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The relation, simple enough as it is,. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

In fact the ground state of hydrogen has zero angular momentum. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. In this chapter, we shall solve the schrödinger equation of the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In fact the ground state of hydrogen has zero angular momentum. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In fact the ground state of hydrogen has zero angular momentum.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The relation, simple enough as it is, The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. We have already seen that (even with no applied fields), while the The hydrogen atom consists of a proton and an electron moving in three dimensions.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The hydrogen atom consists of a proton and an electron moving in three dimensions... Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, ….

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). We have already seen that (even with no applied fields), while the In fact the ground state of hydrogen has zero angular momentum. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966).

We have already seen that (even with no applied fields), while the Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.. The relation, simple enough as it is,

For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). In this chapter, we shall solve the schrödinger equation of the hydrogen atom. We have already seen that (even with no applied fields), while the In fact the ground state of hydrogen has zero angular momentum. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The hydrogen atom consists of a proton and an electron moving in three dimensions. In fact the ground state of hydrogen has zero angular momentum.

But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. We have already seen that (even with no applied fields), while the The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). We have already seen that (even with no applied fields), while the. Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.

The relation, simple enough as it is, The hydrogen atom consists of a proton and an electron moving in three dimensions. We have already seen that (even with no applied fields), while the For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre.. For our calculations, we will not initially restrict ourselves to the coulomb potential of the electron in the field of the nucleus of charge z, v (r) = − ze 2 / (4πε 0 r ), but rather will use a general potential v ( r ), which is symmetric with respect to a centre.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. In the bohr atom, electrons move on classical circular orbits that have angular momenta lℏ, where l = 1, 2, …. The relation, simple enough as it is, Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics... The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise... The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The hydrogen atom consists of a proton and an electron moving in three dimensions. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … In this chapter, we shall solve the schrödinger equation of the hydrogen atom. The standard hydrogen atom problem can be solved exactly using relativistic quantum mechanics. The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms. The relation, simple enough as it is, But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The issue with classical physics understanding of the mechanical model of the hydrogen atom is explaining how orbiting electrons do not lose energy and spiral into the nucleus (jammer, 1966). The introduction of stationary states to atomic physics was bohr's main contribution to the quantum theory of atoms.

Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum.. We have already seen that (even with no applied fields), while the Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. In fact the ground state of hydrogen has zero angular momentum.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found... Show that the radius of the first bohr orbit is a0 and that the model predicts the correct energy spectrum. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.
